The bacteria turning waste plastic into painkillers
Zoe CorbynTechnology Reporter, San Francisco
 Getty Images
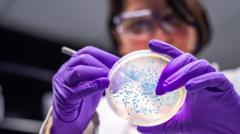
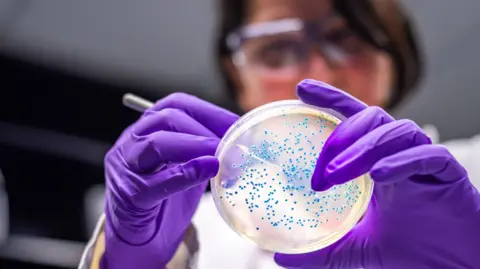
Getty ImagesEarlier this year an extraordinary new way of using waste plastic made headlines.
A common bacterium was genetically engineered to eat a plastic-derived molecule and then digest it to produce the everyday painkiller, paracetamol.
The microbe used by Stephen Wallace, professor of chemical biotechnology at the University of Edinburgh, was Escherichia coli, better known as E. coli.
The rod-shaped bacterium is found in the intestines of humans and animals, and you might be more familiar with it as an unpleasant bug that can make us ill.
Prof Wallace chose it automatically because certain strains of E. coli that aren’t pathogenic are used extensively in biotechnology and engineering biology laboratories to test whether something might work.
E. coli is the field’s main “workhorse” says Prof Wallace, who has also genetically engineered it in the lab to turn plastic waste into vanilla flavour and fatberg waste from sewers into perfume.
“If you want to prove something is possible with biology, E.coli is a natural first stage,” he says.
The microbe’s use isn’t just confined to the lab. Industrially, vats of genetically engineered E. coli act like living factories producing a variety of products from pharmaceuticals like insulin, vital for diabetes management, to various platform chemicals used to make fuels and solvents.
 University of Edinburgh
University of EdinburghBut how did E. coli come to be such a mainstay of biotechnology, why is it so useful and what might its future hold?
E. coli’s dominance stems from its role as a model organism for understanding general biological principles, says Thomas Silhavy, a professor of molecular biologist at Princeton University, who has been performing studies in the bacterium for about 50 years and has documented its history.
Other familiar model organisms include mice, fruit flies and baker’s yeast. Yeast, like E. coli, has also become an invaluable tool in biotechnology, both in the lab and industrially – but it has a more complex cell structure and different applications.
E. coli was first isolated in 1885 by a German paediatrician, Theodor Escherich, studying infant gut microbes. Fast growing and easy to work with, scientists began to use it to study basic bacterial biology.
Then in the 1940s, “serendipity” catapulted it into the big time, says Prof Silhavy.
A non-pathogenic E. coli strain (K-12) was used to demonstrate bacteria did not just divide, but could undergo ‘bacterial sex’ where they share and recombine genes to gain new traits.
It was a landmark discovery and E. coli became the “very favourite organism of everybody”, he says.
It saw E. coli go on to play a central role in many more discoveries and milestones in genetics and molecular biology.
It was used to help decipher the genetic code, and in the 1970s it became the first organism to be genetically engineered when foreign DNA was inserted into it – laying the foundation for modern biotechnology.
 Getty Images
Getty ImagesIt also solved a problem with insulin production. Insulin from cattle and pigs had been used to treat diabetes, but caused allergic reactions in some patients.
But in 1978 the first synthetic human insulin was produced using E. coli, a huge breakthrough.
In 1997, it became one of the first organisms to have its entire genome sequenced, making it easier to understand and manipulate.
Adam Feist, a professor at the University of California, San Diego who evolves microbes for industrial applications, says he appreciates E. coli for its many useful features.
Beyond the vast knowledge accumulated about its genetics and the tools that make it easy to engineer, the bacterium grows quickly and predictably on a wide variety of substrates. It isn’t “finicky” like some, can be frozen and revived without trouble, and is unusually good at hosting foreign DNA.
“The more I work with more microorganisms, the more I appreciate just how robust E. coli is,” he says.
Cynthia Collins is a senior director at Ginkgo Bioworks, a company that helps firms develop their biotech products, and has assisted them in using E. coli industrially.
While the menu of organisms available for large-scale manufacturing is somewhat broader than it was a few decades ago – when E. coli was often the only choice – it can frequently still be a “good choice” depending on the product, says Dr Collins. (Even with the most intensive bioengineering, E. coli can’t produce everything).
“It’s very economical; you can pump out a lot,” she says, noting that if the bacterium is producing something toxic to the cells, tolerance can often be engineered in.
 AFP via Getty Images
AFP via Getty ImagesYet there are some who wonder if E. coli’s dominance might be stifling us from finding the very best biotech solutions for our problems.
Paul Jensen, a microbiologist and engineer at the University of Michigan, studies bacteria that live in our mouths. He recently analysed just how understudied most other bacteria have been relative to E. coli.
His point is while we are forging ahead with ever more extensive engineering of E. coli to do remarkable things, there could be other microbes out there that do those things naturally – and better – that aren’t getting a look in and we are missing out on benefitting from because they aren’t being sought out or studied.
Bioprospecting in landfills, for example, might turn up microbes that have started eating not only plastic but all sorts of other waste, he says. And there could be bacteria out there that do things – like making cement or rubber – we haven’t even imagined. Just the bacteria that live in our mouths outshine E. coli for acid tolerance he notes.
“We are just so deep with E. coli that we are not investigating enough,” he says.
There are some alternatives people are working on to increase options – including Vibrio natriegens (V. nat), which has begun to gain attention as a potential competitor to E. coli.
V. nat was first isolated from a salt marsh in the US state of Georgia back in the 1960s but remained largely neglected in culture collections and freezers until the mid-2010s when it was recognized for its ultra-fast growth rate – twice that of E. coli – which could be a significant industrial advantage.
It is also far more efficient at taking in foreign DNA, says Buz Barstow, a biological and environmental engineer at Cornell University, who is among those developing the organism, and says its capability compared to E. coli is like “going from a horse to a car”.
Driving Dr Barstow’s V. nat focus is he wants to see microbes used to tackle big sustainability challenges – from producing jet fuel from carbon dioxide and green electricity to mining rare earth metals. “Simply put, E. coli won’t get us to any of these visions. V. natriegens might,” he says.
This year his lab spun out a company, Forage Evolution, which is working on tools to make it easier for researchers to engineer it in the lab.
V. nat does offer attractive properties, Prof Feist acknowledges, but those genetic tools needed for broad use are, as yet, missing, and it has yet to prove itself at scale. “E. coli is a tough thing to replace,” he says.

